Doctor-Supervised Injectable Peptide Weight Loss Programs
Doctor‑supervised pharmacologic weight loss relies on prescription medicines—most often injectable peptides—to reduce appetite, slow gastric emptying, and help stabilize metabolism under a clinician’s care. This guide walks you through how the leading injectables work, who typically qualifies, and how a structured, physician‑led program coordinates labs, careful dose titration, and ongoing monitoring so treatment stays safe and effective. Many patients encounter variable weight‑loss results, medication side effects, and insurance questions; pairing evidence‑based drug selection with close follow‑up helps clinicians and patients navigate those issues together. Below we summarize the most effective injectable agents (including GLP‑1 and GLP‑1/GIP therapies), outline common program steps, share practical side‑effect management tips, compare popular peptides, clarify typical cost and insurance considerations, and explain how to begin care with a supervised clinic. Our goal is clear, research‑grounded explanation so you know how these medicines work, what outcomes are realistic, and what to expect on the patient journey.
Which Weight‑Loss Drugs and Injections Work Best?
The most effective injectables target appetite and metabolic pathways—mainly GLP‑1 receptor agonists and dual GLP‑1/GIP agents. These medicines reduce calorie intake and improve blood‑sugar control. Trials through 2023–2024 show meaningful average weight loss with these drug classes when combined with lifestyle support, which is why they are central to contemporary pharmacologic obesity care. Eligibility typically depends on BMI thresholds and metabolic conditions. Doses are delivered subcutaneously and escalated stepwise to enhance tolerability. Knowing each agent’s profile helps clinicians and patients choose a plan that matches goals, medical history, and side‑effect tolerance.
Semaglutide and tirzepatide are two commonly used injectables with robust trial evidence for clinically meaningful weight loss. Below is a short list of leading agents, why they’re prescribed, and practical notes on their use.
- Semaglutide: A GLP‑1 receptoragonist that markedly reduces appetite and leads to sustained weight loss over months in clinical studies.
- Tirzepatide: A dual GLP‑1/GIP agonist that, in head‑to‑head trials, often produces larger average weight reductions than GLP‑1 alone.
- Adjunct peptides (for example, tesamorelin, naltrexone, sermorelin): Used selectively to target visceral fat, support muscle mass, or modify reward pathways.
All of these medications are given by subcutaneous injection and usually start with a titration phase to reach a maintenance dose that balances benefit and tolerability. The sections that follow describe how semaglutide and tirzepatide work and what patients commonly experience during dose escalation.
How Semaglutide Helps With Weight Loss
Semaglutide is a GLP‑1 receptoragonist that reduces hunger, slows gastric emptying, and improves metabolic signaling. Together these effects lower daily calorie intake and support steady body‑weight reductions. It acts at GLP‑1 receptors in the brain and gut to increase feelings of fullness and reduce appetite, producing gradual, sustained weight loss in trials. Semaglutide is administered once weekly by subcutaneous injection and started with gradual dose increases to limit gastrointestinal side effects and reach an effective maintenance dose. In practice, semaglutide is combined with behavior counseling and metabolic monitoring to maximize benefit and safety.
Semaglutide is a strong option for people who need reliable appetite control and have no contraindications to GLP‑1 therapy. Clinicians individualize titration timing and follow‑up to each patient’s tolerance and response, watching weight trends, glucose markers, and side effects. Understanding how semaglutide complements diet and activity helps set realistic expectations for the pace of weight and metabolic change.
What Tirzepatide Offers for Weight Loss
Tirzepatide targets both GLP‑1 and GIP receptors, producing a dual hormonal effect on appetite and metabolism that often results in greater average weight loss than GLP‑1 drugs alone. The GIP activity appears to amplify weight‑loss magnitude and improve glycemic control for many patients, making tirzepatide especially useful for people with obesity and metabolic disease. It’s given once weekly by subcutaneous injection with graded dose escalation to balance benefit and tolerability; clinicians monitor for gastrointestinal symptoms during titration. Choosing tirzepatide depends on a person’s metabolic profile, treatment goals, and prior response to GLP‑1 therapies.
For patients seeking maximal weight loss or those with type 2 diabetes who want combined glucose and weight benefits, tirzepatide can be an excellent option—assuming the individual risk–benefit assessment supports it. Close follow‑up during dose changes helps maintain adherence and reduces the chance of stopping treatment because of side effects.
GLP‑1 and Dual GIP/GLP‑1 Receptor Agonists: Mechanisms and Clinical Applications
Concise summary of mechanisms and therapeutic uses for GLP‑1 and dual GIP/GLP‑1 receptor agonists. Our research integrity and auditing teams oversee reviews to protect the quality of the scientific evidence.
Mechanisms of action and therapeutic applications of GLP‑1 and dual GIP/GLP‑1 receptor agonists, 2024
How a Doctor‑Supervised Medical Weight Loss Program Functions
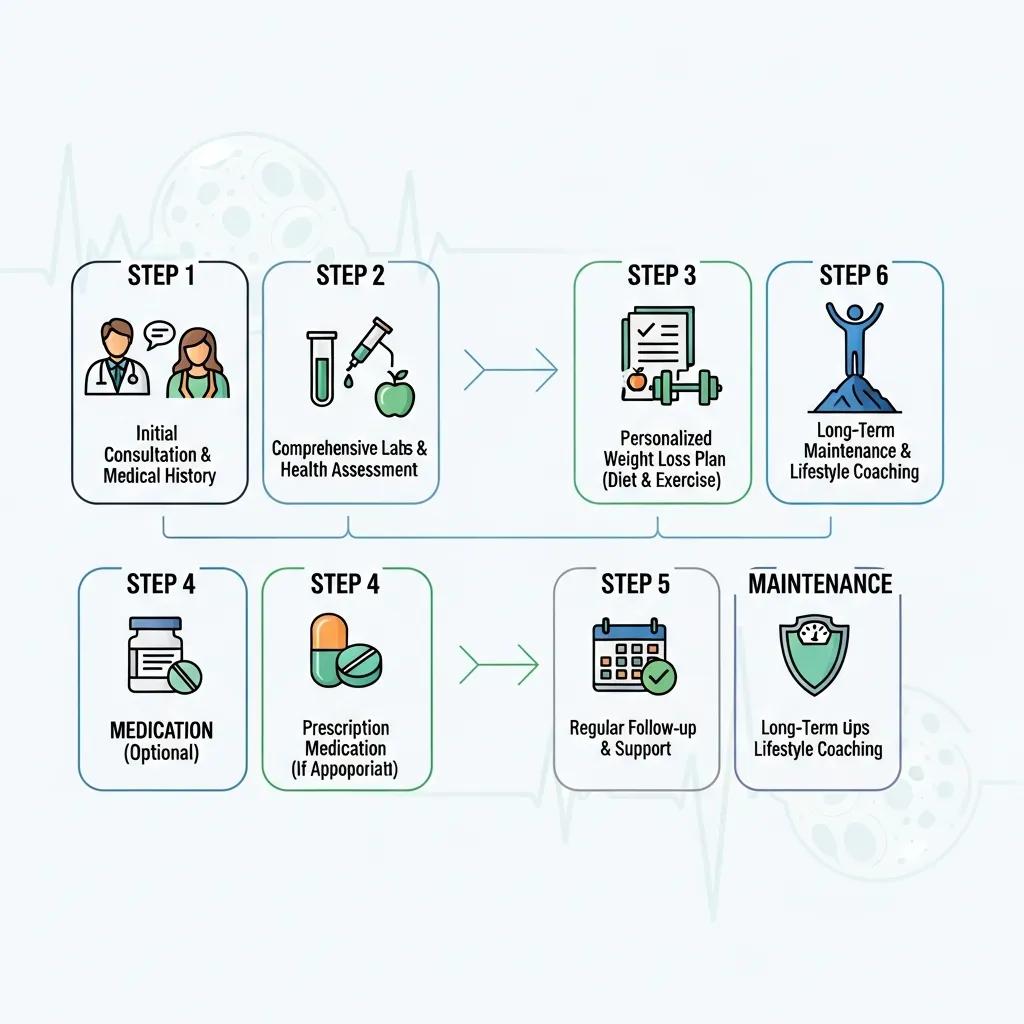
A physician‑supervised weight‑loss program blends a clinical assessment, baseline labs, tailored peptide selection, supervised injection starts, and scheduled monitoring so care stays both safe and productive. The pathway usually begins with a medical evaluation and eligibility screening, followed by labs that identify contraindications and establish metabolic baselines to guide drug choice and dosing. Personalized plans set the injectable agent, titration schedule, optional adjuncts for muscle support, and a follow‑up cadence to track safety and progress. Regular monitoring lets clinicians adjust doses, address side effects early, and reinforce behavior changes that support adherence and metabolic improvement.
The table below outlines common program steps, what happens at each stage, and the primary patient benefit—so the process is transparent for prospective patients and clinicians.
| Program Step | What Is Done | Purpose / Patient Benefit |
|---|---|---|
| Initial consultation | Comprehensive medical history, medication review, and risk screening | Determines eligibility and identifies key safety considerations for peptide therapy |
| Baseline labs | Metabolic panel, A1c, liver tests, and other targeted studies | Provides objective baselines and screens for contraindications |
| Personalized plan | Selection of drug, dosing schedule, and optional adjuncts for muscle support | Aligns therapy with goals and reduces the chance of adverse outcomes |
| Injection initiation & training | First doses supervised and injection technique demonstrated | Ensures safe administration and early tolerance checks |
| Follow-up & monitoring | Regular check‑ins and labs every three months or sooner as needed | Tracks progress, guides dose adjustments, and manages side effects proactively |
This structured pathway reduces uncertainty and improves safety compared with unsupervised use, while clarifying roles for both clinician and patient throughout treatment. Below we describe common elements of an individualized peptide injection plan and how medical history and labs inform those choices.
What Goes Into a Personalized Peptide Injection Plan?
A tailored peptide plan spells out the chosen injectable, a titration timeline, optional adjunct peptides for muscle or visceral‑fat goals, and scheduled monitoring checkpoints. Clinicians choose a GLP‑1 versus a GLP‑1/GIP agent based on BMI, metabolic comorbidities, prior medication history, and the patient’s goals, and they adjust titration speed to reduce gastrointestinal side effects. Adjuncts—when appropriate—support lean mass or metabolic rate without undermining weight‑loss effects; labs guide safe combinations. Patients receive clear written goals, expected timelines, and criteria for dose escalation or stopping therapy, so they know when to contact their care team.
This individualized planning improves adherence and lowers the risk of adverse events through proactive education and follow‑up—which is why ongoing monitoring matters for long‑term success.
How Ongoing Monitoring Improves Outcomes
Regular monitoring—weight checks, lab surveillance, and symptom review—lets clinicians fine‑tune dosing, detect early warning signs, and reinforce behaviors that enhance medication effectiveness. Routine reviews every three months (or sooner as needed) reveal metabolic gains or lab changes that guide whether therapy should continue or be modified. Frequent touchpoints also increase accountability and adherence. Monitoring helps manage plateaus by combining pharmacologic tweaks with lifestyle support and, when appropriate, adjunct therapies. Together, these steps reduce risk and support durable weight‑loss maintenance by aligning treatment decisions with patient progress and safety data.
Active monitoring builds a collaborative relationship where dose changes and supportive measures happen quickly—preventing minor problems from becoming reasons to stop treatment.
What Side Effects Are Common and How Are They Managed?
Injectable weight‑loss medications most commonly cause gastrointestinal symptoms, injection‑site reactions, and—less often—events needing urgent evaluation. Most adverse effects are manageable when clinicians anticipate them. Early reactions typically include nausea, vomiting, diarrhea, or constipation related to delayed gastric emptying and appetite changes. Practical strategies—slow titration, targeted dietary adjustments, short‑term antiemetics when appropriate, and clear counseling on expectations—improve tolerability and help patients remain on therapy. Knowing when to seek urgent care—severe abdominal pain, signs of pancreatitis, or gallbladder symptoms—allows prompt intervention and safer treatment.
- Nausea and vomiting: Start at a low dose and increase slowly; eat smaller, bland meals and consider short‑term antiemetics under clinician guidance.
- Diarrhea or constipation: Adjust fiber and fluids, use stool‑forming or softening agents as recommended, and reassess titration if needed.
- Injection‑site reactions: Rotate sites, use correct technique, and apply cold packs; most local reactions resolve without stopping therapy.
These measures lower early discontinuation and improve real‑world results. The next section compares side‑effect patterns between semaglutide and tirzepatide so patients and clinicians can weigh tolerability differences.
What Side Effects Are Typical with Semaglutide and Tirzepatide?
Both semaglutide and tirzepatide commonly cause gastrointestinal symptoms—nausea, vomiting, diarrhea, and constipation—with frequency tied to dose and how quickly it’s raised. Tirzepatide’s dual activity can produce stronger effects and, for some people, a higher GI burden. Trial data show most GI events are temporary and cluster around the dose‑escalation phase, underscoring the value of gradual titration. Less frequent but important risks include gallbladder‑related events and rare signals that require vigilance; clinicians monitor symptoms and labs to detect these early. When side effects persist, options include dose adjustment, a temporary hold, or switching agents after a risk–benefit discussion.
Understanding expected timelines and relative frequencies helps clinicians design titration plans that balance potency with tolerability. The next section outlines specific mitigation tactics.
How Can Side Effects Be Managed During Treatment?
Managing side effects combines medication strategy, diet, and behavior: slow dose increases, small frequent meals, good hydration, and short‑term supportive meds for nausea when indicated. Thoughtful titration reduces early GI intensity, and dietary counseling—such as avoiding high‑fat meals that worsen nausea—improves comfort. If symptoms persist, clinicians may pause escalation, reduce the dose, or switch therapies based on labs and a risk–benefit review. Clear communication and early check‑ins move management from reactive to proactive, supporting adherence and better long‑term results.
With these practical measures, many patients continue therapy and achieve meaningful weight loss while keeping discomfort manageable.
How Do Semaglutide, Tirzepatide and Other Peptides Compare?
Comparing injectable options means looking at mechanisms, typical efficacy ranges, and administration so clinicians and patients can match therapy to clinical goals. Semaglutide (a GLP‑1) primarily suppresses appetite and slows gastric emptying; tirzepatide (GLP‑1 + GIP) adds GIP activity that can amplify weight loss and metabolic benefit. Other peptides—like tesamorelin for visceral fat, naltrexone for reward‑pathway effects, and sermorelin for growth‑hormone axis support—can be complementary when used thoughtfully. The table below gives a concise comparison to aid quick decision‑making.
| Drug | Mechanism / Drug Class | Typical Weight Loss & Administration |
|---|---|---|
| Semaglutide | GLP‑1 receptor agonist | Consistent, clinically significant weight loss in trials; once‑weekly subcutaneous injection with stepwise titration |
| Tirzepatide | Dual GLP‑1/GIP agonist | Often larger average weight loss than GLP‑1 alone; once‑weekly injection with graded dosing |
| Tesamorelin | GHRH analog (targets visceral fat) | Reduces visceral adiposity in select patients; daily or clinician‑directed schedule |
How Do Mechanism and Efficacy Differ?
GLP‑1 drugs like semaglutide reduce appetite and slow gastric emptying to produce steady weight loss over weeks to months. GLP‑1/GIP dual agonists such as tirzepatide engage both incretin pathways to enhance weight and glycemic responses. Head‑to‑head data in recent years often show tirzepatide achieves greater average weight loss than semaglutide at comparable time points, but individual responses vary and side‑effect profiles influence real‑world choices. Clinically, GLP‑1/GIP agents may be preferred when maximal weight reduction is the goal or when significant metabolic disease is present; GLP‑1 monotherapy can be chosen when a gentler tolerability profile is desired. Matching the drug mechanism to the patient’s phenotype—such as central adiposity or marked hyperglycemia—supports better individualized outcomes.
Final decisions should weigh patient goals, side‑effect risk, and the clinic’s monitoring capacity to select the best injectable for each person.
Which Additional Peptides Might Be Offered?
Beyond GLP‑1 and GLP‑1/GIP agents, clinics may offer peptides such as tesamorelin for visceral fat reduction, naltrexone in combination regimens to blunt reward‑driven eating, and sermorelin to help preserve lean mass via growth‑hormone‑axis support in select patients. Each has a targeted role: tesamorelin for central adiposity, naltrexone for reward modulation, and sermorelin for anabolic support when maintaining muscle is a priority during calorie reduction. These adjuncts are combined cautiously based on labs, goals, and contraindications to avoid interactions and protect safety. High‑quality programs prioritize pharmaceutical‑grade sourcing and lab verification to safeguard product integrity and patient safety when agents are combined.
What Will Treatment Cost and What About Insurance?
Out‑of‑pocket costs for injectable weight‑loss medications vary widely by drug, dose, clinic services, and insurance coverage—monthly medication costs can range from moderate to substantial depending on brand and dosing. Insurers are more likely to cover these drugs when prescribed for diabetes rather than for weight alone, and many payers require prior authorization for obesity indications, so benefit checks are an important early step. Program fees—covering evaluation, labs, injection training, and monitoring—are separate from medication costs and affect total expense. Patients can explore FSA/HSA use, payment plans, and bundled pricing that some clinics offer to improve affordability.
The table below gives a clear snapshot of typical cost ranges by service and the likelihood of insurance coverage to help patients plan financially.
| Service / Injection | Typical Cost Range | Insurance Coverage Likelihood / Notes |
|---|---|---|
| GLP‑1 / GLP‑1GIP medication (monthly) | Varies by agent and dose; can range from several hundred dollars to over $1,000 | More likely covered when prescribed for diabetes; prior authorization often required |
| Clinic program fees (initial + monitoring) | Varies by clinic package | Usually out‑of‑pocket; some clinics offer payment options |
| Adjunct peptides (per course) | Varies by peptide and regimen | Typically out‑of‑pocket; coverage uncommon for obesity indications |
Clear cost information helps patients compare options and plan. Some clinics also publish transparent pricing and satisfaction guarantees to reduce uncertainty for people new to injectable therapy.
UCO Medical Clinic, in Hallandale Beach, Florida, operates a doctor‑supervised weight‑loss program that emphasizes pharmaceutical‑grade peptides sourced from licensed 503B pharmacies with third‑party testing, individualized injection plans, and scheduled monitoring with check‑ins every three months or sooner. We offer virtual consultations for convenience and a money‑back guarantee after the first injection, reflecting transparent pricing and our commitment to patient satisfaction. These program features show how clinic‑level quality controls and clear policies can simplify treatment decisions for prospective patients.
How Much Do Medical Weight‑Loss Injections Usually Cost?
Monthly medication costs and program fees vary by agent, dose, and the level of clinical support provided; branded injectables are often the largest ongoing cost. Program fees usually cover the initial evaluation, baseline labs, injection training, and follow‑up; higher‑touch programs with more frequent monitoring or extra services raise total cost but may improve safety and adherence. Key cost drivers include brand versus compounded sourcing, dosing frequency, and use of adjunct peptides. Ask your clinic for a detailed breakdown of medication versus service fees and about payment supports such as FSA/HSA or plans to manage out‑of‑pocket expenses.
Receiving a transparent, written fee schedule before starting helps set expectations and avoid surprises.
What Insurance Coverage Can Patients Expect?
Insurance coverage for weight‑loss medications depends on payer policy, the clinical indication, and formulary status—coverage is more likely when the drug is prescribed for diabetes or another covered metabolic condition. Many insurers require prior authorization, documentation of medical necessity, and step‑therapy steps before approving obesity indications. Clinics often assist by verifying benefits, submitting prior authorizations, and supporting appeals to improve coverage chances. Patients should request an explanation of benefits and work with their clinic to understand documentation and potential out‑of‑pocket responsibilities.
Proactive benefit checks and clinician‑led appeals can materially affect access and affordability for eligible patients.
How Do Patients Begin a Doctor‑Supervised Peptide Injection Program?
Care usually begins with an initial consultation—virtual or in‑person—where the clinician reviews your medical history, discusses goals, and orders baseline labs to assess safety and guide drug selection. The visit covers medication options, expected timelines, contraindication screening, and an agreed monitoring schedule. Virtual visits increase access and convenience for many steps, while some elements—like a supervised first dose or a detailed physical exam—may require an on‑site appointment. You’ll receive clear next steps, informed‑consent materials, and a titration and monitoring timetable so you know what to expect in the early months of care.
The checklist below lists what to bring and expect at your first appointment to make the process smoother.
- Bring a current medication list and relevant medical records: Gives clinicians the context needed for safe prescribing.
- Expect baseline labs to be ordered or reviewed: Establishes metabolic baselines and screens for contraindications.
- Discuss treatment goals and preferences: Helps align drug choice and monitoring with what matters to you.
- Review consent, side‑effect expectations, and the follow‑up schedule: Ensures informed decision‑making and a safety plan.
What Happens During the Initial Consultation?
At the initial visit, clinicians take a focused medical history, review current medications, screen for contraindications, and discuss weight‑loss goals to determine whether injectable therapy is appropriate and safe. Baseline labs—such as metabolic panels and targeted tests—are ordered to give objective data that guide drug selection and monitoring frequency. The clinician explains how proposed injectables work, typical timelines for weight changes, common side effects, and mitigation strategies, and obtains informed consent before starting therapy. The visit concludes with a clear plan for titration, scheduled follow‑ups, and instructions for when to seek urgent care—prioritizing safety and aligned expectations.
How Do Virtual Consultations Support Ongoing Care?
Virtual consultations provide convenient access to clinician evaluation, medication planning, and routine follow‑up—reducing travel barriers and supporting continuity. Remote visits work well for history‑taking, counseling, titration decisions, and symptom checks; labs can be coordinated locally and reviewed by your treating clinician. Some steps—such as a supervised first injection or complex in‑person assessments—may still require office visits, but most follow‑ups and dose adjustments can be handled virtually. Virtual care pathways paired with scheduled lab monitoring and clear escalation protocols create a flexible, safe model for ongoing peptide therapy.
For patients who want doctor‑led care with virtual access and pharmaceutical‑quality medications, clinician‑run programs that emphasize verified sourcing and regular monitoring offer both convenience and strong safety oversight—helping you start and stay on track through the early, critical months of treatment.
Frequently Asked Questions
What should I expect during the initial consultation for a weight loss program?
Expect a thorough medical history review, medication reconciliation, and a conversation about your goals. The clinician will order baseline labs to check metabolic health and rule out contraindications. You’ll learn how the proposed injectable works, the typical timeline for results, common side effects, and strategies to manage them. This visit ensures you have the information needed to give informed consent and to begin a safe, individualized plan.
How do I know if I am a candidate for weight loss injections?
Eligibility usually depends on BMI thresholds and the presence of metabolic conditions, but candidacy is individualized. The initial evaluation considers prior weight‑loss attempts, current health, and your goals to determine whether injectable therapy is appropriate. If you meet clinical criteria, your clinician will discuss suitable, tailored options.
Are there any lifestyle changes I need to make while on weight loss injections?
Yes—lifestyle changes increase medication benefits. We recommend a balanced diet, regular physical activity, and behavioral strategies to support long‑term results. Clinicians provide practical guidance on diet and exercise that fits your preferences and abilities to help maximize outcomes and overall health.
What happens if I experience side effects from the injections?
If you have side effects, contact your clinician promptly. Common issues—like nausea or diarrhea—are often managed with slower titration and dietary adjustments. Your clinician may suggest short‑term supportive medications or change the dosing plan. Regular monitoring and open communication ensure side effects are addressed early so you can continue treatment safely.
How long does it take to see results from weight loss injections?
Responses vary, but many people notice early weight changes within a few weeks, with more substantial results over several months. Progress depends on metabolism, adherence to the plan, and lifestyle changes. Regular follow‑ups let clinicians track progress and adjust the plan to optimize outcomes.
Can I continue my current medications while on weight loss injections?
Often yes, but this requires clinical review. Some medications can interact with weight‑loss drugs or affect safety and efficacy. Bring a complete medication list to your consultation so your clinician can assess compatibility and make any necessary adjustments.
What should I do if I want to stop the weight loss program?
If you decide to stop, discuss it with your clinician first. They’ll advise how to discontinue safely and help you develop a plan to maintain progress. Stopping without clinical guidance can lead to weight regain or other issues; your clinician can recommend alternatives for long‑term health.
Conclusion
Doctor‑supervised programs using injectable medications such as semaglutide and tirzepatide offer a structured, evidence‑based path to meaningful weight loss and improved metabolic health. These programs combine personalized treatment plans, careful monitoring, and practical strategies to maximize safety and results. Understanding the process helps you make an informed choice about your care. To take the next step, schedule a consultation with our experienced team and we’ll guide you through a safe, personalized plan.
